Can You Have Cacna1a and Not Know It
- Case report
- Open up Access
- Published:
Ii singled-out phenotypes, hemiplegic migraine and episodic Clutter type two, acquired past a novel common CACNA1A variant
BMC Neurology volume 20, Article number:155 (2020) Cite this article
Abstruse
Background
To investigate the genetic and ecology factors responsible for phenotype variability in a family unit conveying a novel CACNA1A missense mutation. Mutations in the CACNA1A gene were identified as responsible for at to the lowest degree three autosomal ascendant disorders: FHM1 (Familial Hemiplegic Migraine), EA2 (Episodic Clutter type 2), and SCA6 (Spinocerebellar Ataxia type half dozen). Overlapping clinical features inside individuals of some families sharing the aforementioned CACNA1A mutation are not exceptional. Conversely, reports with distinct phenotypes within the same family associated with a common CACNA1A mutation are very rare.
Example presentation
A clinical, molecular, neuroradiological, neuropsychological, and neurophysiological study was carried out in proband and his carrier mother. The new heterozygous missense variant c.4262G > A (p.Arg1421Gln) in the CACNA1A factor was detected in the 2 affected family members. The proband showed a complex clinical presentation characterized by developmental filibuster, poor motor coordination, hemiplegic migraine attacks, behavioral dysregulation, and EEG abnormalities. The mother showed typical episodic clutter attacks during infancy with no other comorbidities and mild cerebellar signs at present neurological evaluation.
Conclusions
The proband and his mother exhibit two distinct clinical phenotypes. It tin can be hypothesized that other unknown modifying genes and/or environmental factors may cooperate to generate the wide intrafamilial variability.
Background
Hemiplegic Migraine (HM) is a rare migraine variant characterized past specific aura signs including some caste of hemiparesis and/or i-sided weakness. It may include sensory, visual or language impairment during aureola. 2 forms of HM take been recognized: familial hemiplegic migraine (FHM MIM #301011), in which at to the lowest degree a relative has migraine aura signs with the same clinical features, and sporadic hemiplegic migraine (SHM) with no recognizable familial history of hemiplegic migraine. FHM is an autosomal dominant inherited disorder caused by mutations in ion transporters encoding several genes: CACNA1A mutations cause FHM type 1 (FHM1, MIM:141500), ATP1A2 mutations cause FHM blazon 2, SCNA1 mutations cause FHM type 3 (ICHD-3beta, 2013) [1]. Episodic clutter blazon 2 (EA2, MIM: 108500) is a paroxysmal neurological dysfunction of cerebellum lasting minutes to hours that includes symptoms similar clutter, nausea,vomiting, vertigo, diplopia, nystagmus, dysarthria, tinnitus, headache, and hemiplegia. Historic period of onset ranges between the first and the 2d decade of life, prevalence rate is unknown. The EA2 attacks can exist triggered past some stressors such as alcohol, caffeine, physical and mental stress and postural changes, and relieved past residuum and slumber. Neurological examination between attacks may be normal, just some patients may develop ataxia and nystagmus and progressive cerebellar atrophy on MRI. EA2 is an autosomal dominant disorder acquired by mutation in the CACNA1A gene [2]. Mutations of the CACNA1A gene, located on chromosome 19p13.xiii, coding for the α1 pore –forming subunit of the voltage-gated calcium aqueduct P/Q blazon (Cav2.1), were identified as responsible for three autosomal dominant disorders with incomplete penetrance: FHM1, EA2, and spinocerebellar clutter type six (SCA6, MIM:183086) [3, 4]. However, overlapping clinical features described within some families with the same CACNA1A mutation are not infrequent [5,6,7,8]. Conversely, Pradotto reported singled-out phenotypes, ranging from EA2 to SCA6, within the same family associated with a common CACNA1A mutation [9]. We study a kid with a clinical phenotype characterized past some hemiplegic migraine attacks and neurodevelopmental disorder associated with a novel heterozygous c.4262G > A CACNA1A (NM_023035.2) variant. The aforementioned genetic aberration was detected only in his mother with clinical history of a distinct infantile EA2 without migraine.
Case presentation
This study was approved by the ideals committee Palermo one of the "Paolo Giaccone" University Infirmary of Palermo, Italy. Written informed consent for participate and for publication was obtained from each of the family members tested. The proband is a 7 year old child, the offset of two siblings born to apparently good for you unrelated parents at the end of a pregnancy undertaken by an emergency caesarean section, post-obit a gestational menstruum complicated with some bleeding events from the seventh calendar month.
His birth weight was 3900 g and his clinical postnatal class was uneventful. His developmental milestones have been delayed; he vocalized undifferentiated nasal sounds at 12 months, spoke the first word at two years and at xiii months he walked unsupported. He is currently under speech and neuropsychomotor therapy.
At the historic period of iv years and eight months, he was referred to our Child Neuropsychiatric Department for unilateral frontal headache attacks associated with pallor, crying, discomfort in the left lower limb, left-sided weakness, mostly in the left lower limb, leading to unsteady posture. The clinical events, started from iv years of historic period, occurred almost every day in both wakefulness and slumber, lasted 10 to30 minutes, and spontaneously remitted after resting.
On admission the neurological examination showed a moderate motor and coordination impairment. The cognitive and behavioral evaluation revealed a severe deficit in behavioral regulation (restlessness, poor command of emotional drives with ambitious behavior, poor waiting and following rules abilities) and impaired language with severe phonetic-phonological failings.
Wakefulness and sleep EEG displayed recurrent generalized 3 Hz regular spike-moving ridge circuitous discharges lasting 1 to 3 s without associated clinical events (Fig. 1).
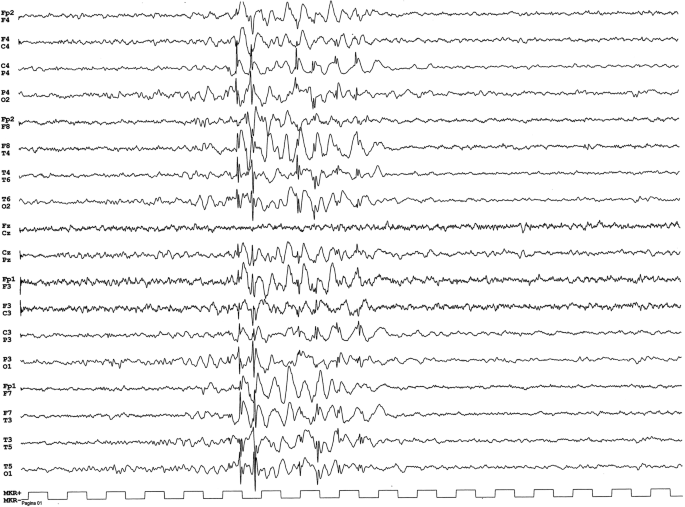
The EEG displayed recurrent generalized iii Hz regular fasten-wave complex discharges lasting 1to 3 southward without associated clinical events
A handling of valproic acid with increasing doses up to thirty mg∕ kg/die was started. The electroclinical follow-up displayed a hitting disappearance of EEG abnormalities associated with a decrease of hemiplegic migraine attacks and a mild comeback of behavioral symptoms.
The latest clinical evaluations confirmed the disappearance of the generalized 3 Hz spike-moving ridge discharges, showed a decrease of hemiplegic migraine attacks, unchanged motor abnormalities and a worsening of behavioral problems that required an antipsychotic drug in add-on to valproic acid.
The child underwent a comprehensive neuropsychological assessment using a bombardment of tests standardized for the Italian population (WISC-IV, NEPSY-II, Vineland ABS-Ii; references were reported in "supplementary textile") to evaluate intellectual, cerebral, and behavioral functions. The formal assessment showed an extremely depression overall intellectual ability (IQ = 44) with deadline to average conceptual reasoning and problem-solving skills (PRI 72-88). The operation on measures of expressive vocabulary and lexical knowledge were in the borderline average range. The comprehension of instructions was impaired. The performance on motor dexterity and visuomotor precision was dumb; visuospatial and constructional tasks scored in the average- low average range. The retention tasks for faces and for names fell in the average and below average respectively. The performances on memory for designs and a story retentivity tasks were impaired in both immediate and delayed gratis think. Attention was dumb on auditory tasks and in average range on visual tasks. Executive functions were impaired on inhibition, verbal fluency, and planning tasks. Social cognition (ToM and affect recognition from faces tasks) was dumb (Tabular array 1).
The child showed difficulties in emotion regulation including easy frustration, temper tantrums, oppositional behaviors, verbally and physically aggressive behavior.
MRI investigation, performed at 5 years of historic period, did not show structural abnormalities. The more detailed family clinical history highlighted some paroxysmal events in mother childhood with onset at eight years of historic period characterized past sudden dramatic gait unsteadiness, vertigo, nausea and sometimes airsickness. These attacks occurred several times a week and lasted for hours, they were triggered by exertion and postural changes and relieved past remainder and sleep. At the age of 15 interictal neurological and vestibular evaluation, and a cranial CT scan were not informative regarding the disorder crusade. Although no handling was recommended, the frequency of attacks graduallydecreased until their remission. In addition, the female parent firmly denied a family history of FHM1 or EA2 attacks and SCA6. A recent neurological evaluation revealed nystagmus by lateral gaze, moderate fine motor harm, dysdiadochokinesia. The intellectual cess showed an extremely low overall intellectual ability (WAIS Iv IQ = 49). General adaptive functioning was in the average compared to age matched peers (Vineland II: Adaptive Behavior Composite = xc) and she engages in a work activity and takes intendance of her family. The clinical and EEgraphic cess, extended to the sis and the male parent of proband, showed neither migraine and / or cerebellar symptoms, nor cognitive and behavioral impairment, nor the 3 Hz SW pattern found in the patient.
A next generation sequencing panel, exploring 45 genes associated with epileptic encephalopathy (for more details come across the "supplementary cloth"), performed in the proband and his family members, showed a new heterozygous missense variant, c.4262G > A (p.Arg1421Gln) in the CACNA1A gene (NM_023035.2), [Chr19(GRCh37):g.13372264C > T], inherited from his mother, never described in literature and not reported in gnomAD and ExAC database (Fig. 2).
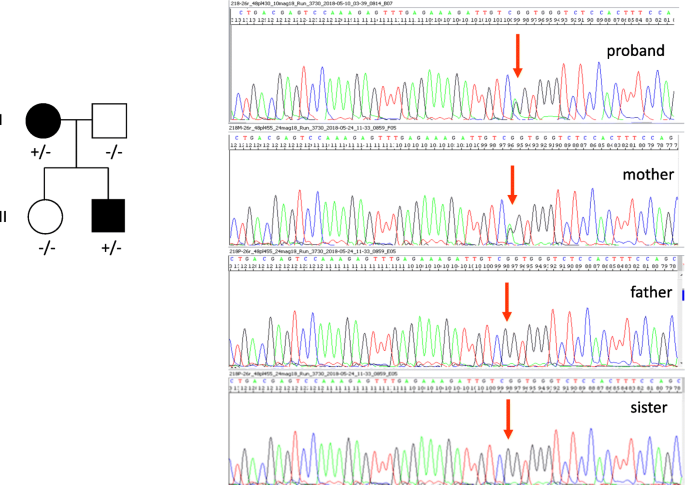
The novel mutation at c.4262G > A, p.(Arg1421Gln) in the CACNA1A gene was found in the proband and his mother
This variant was non been found in his healthy male parent and his healthy sister. This substitution causes the replacement of a positively charged arginine with a neutral not polar glutamine and involves a highly conserved residue located in the extracellular pore loop connecting S5 and S6, likely altering the calcium aqueduct structure at this functionally critical region. A computer-based analysis, able to predict the issue of amino acid change on poly peptide structure and function by using iii dissimilar algorithms, PolyPhen2(http://genetics.bwh.harvard.edu/pph2/), Mutation taster http://world wide web.mutationtaster.org/, and HSF (https://umd.be › HSF) respectively, indicated a possible deleterious effect on protein function (mayhap damaging -score 0,6- for PolyPhen2, disease causing -score 43- for Mutation taster, and potential amending of splicing for HSF). In improver, the variant, located very close to the exon-intron purlieus, could atomic number 82 to an abnormal splicing resulting in aberrant transcripts (Alamut visual ese predictions: Splicing predictions at nearest natural junction predicted modify at donor site 1 bps downstream: − 64.2% MaxEnt: -81.two%, NNSPLICE: -98.9%, HSF: − 12.half dozen%).
Discussion and conclusions
The neuronal CaV channels control several cellular functions and are involved in some neurological and psychiatric disorders [x].
The Cav2.ane channels are localized at presynaptic nerve terminals where the depolarization induces voltage-dependent calcium influx mediating the neurotransmitter release. They are expressed in all brain regions including the thalamocortical system, where P/Q-type VGCCs back up the production of characteristic γ-ring oscillations considered to exist a functional prerequisite to cerebral states, simply they are particularly expressed in Purkinje and granule cells [11, 12]. The identification of the pathogenic office of CACNA1A mutations in some disorders such as EA2, FHM1, and SCA6 suggested that these may be considered allelic disorders [3, 4]. Earlier it was hypothesized that the mutations inducing loss-of-role were associated with EA2, missense mutations resulting in gain-of-part acquired FHM1, and aberrant CAG expansions were related with SCA6 [3, 4]. Merely overlapping features, including typical and uncommon symptoms of mentioned disorders, have been reported among the members of the same family [5,6,seven,8]. Recently, a common CACNA1A mutation with distinct phenotypes, ranging from epileptic encephalopathy, EA2 to SCA6, was also institute within the same family, suggesting that several factors could play a role in the phenotypic variability [9, 13].
Our affected family unit members share the same heterozygous c.4262G > A CACNA1A nucleotide substitution, located very close to the exon-intron purlieus, which leads to the p.Arg1421Gln missense mutation. This variant is non included in the gnomAD browser and never identified in patients with FHM1, EA2, SCA6 with whom, therefore, a comparing is impossible. Nevertheless, information technology has been reported that well-nigh FHM1 and EA2 missense mutations produce substitutions of conserved amino acids (oft arginine) clustering in important functional regions of the Cavii.ane aqueduct including the pore lining (FHM1), the voltage sensors (FHM1), and the S5–S6 linkers (EA2) [12, fourteen, 15]. In addition, the p.Arg1421Gln variant segregating in all our afflicted family members and sparing the asymptomatic father and sister enhances its pathogenic relevance. The two patients showed two singled-out phenotypes, the mother exhibited the typical EA2 without other comorbidities, and her son adult a more complex clinical feature characterized by hemiplegic migraine attacks, developmental filibuster, poor developmental motion coordination, and behavioral dysregulation. Since no relative has migraine aura symptoms, our child'due south clinical feature could be classified as SHM with early on onset. This co-occurrence in CACNA1A mutant patients has been associated with more than severe outcome [sixteen]. Many studies remarked in FHM1, EA2,and SCA6 patients the co-occurrence of behavioral and neuropsychological disorders but the investigations carried out with formal neuropsychological testing are scarce [17,eighteen,nineteen]. Recently, a large case series of 23/44 patients (eleven/23 FHM1,10/23 EA2, 2/23 SCA6), investigated by a formal neuropsychological testing, predominantly showed figural memory, visuoconstructive abilities, and verbal fluency damage. Furthermore, the study revealed that neuropsychological deficits were consistent with history of development filibuster of patients [19]. A case study carried out using a formal neuropsychological assessment reported an harm of semantic fluency and of processing speed, and a mild executive dysfunction [18]. A careful evaluation of our patient using a comprehensive neuropsychological battery revealed a partial agreement with the results of Indelicato and Trahan. Indeed, we pointed out intellectual disability and motor skill disorders but the lack of visual constructive disturbances. This discrepancy could depend more on the higher sensitivity of the WISC IV and NEPSY Two than on the diagnostic tools used in to a higher place studies. We presume that the behavioral symptoms of our patient, irrespective of the lack of cerebellum atrophy, may depend on cerebellar dysfunction, being the Cav2.1 channel greatly expressed in the cerebellum. These behavioral symptoms could be comparable to the clinical features characterizing the well-known cerebellar cerebral affective syndrome [twenty,21,22]. Evolution filibuster of the kid suggests also an impairment of cerebral networks due, besides the cerebellar dysfunction, also to an aberrant hippocampal neurotransmission as documented in knock-in mice model expressing the FHM1 mutation [23].
In addition, the child showed an electroencephalographic pattern characterized by frequent but brusk generalized iii Hz regular spike-wave discharges without evidence of ictal clinical events. However, their disappearance after valproic acrid treatment associated with a mild improvement of attending and behavioral symptoms suggested that the child for an indefinite fourth dimension may accept suffered from "micro absence" and/or a mild cognitive impairment. This finding agrees with the electroclinical design frequently highlighted in EA2 patients carrying a truncating mutation of CACNA1A, suggesting a part of calcium channels in absence epilepsy although the electroclinical pattern in our patient is associated with the FHM1 phenotype [24, 25].
The impressive behavioral characteristic found in our patient and in many subjects previously reported in literature suggests the possibility to further expand the phenotype of CACNA1A mutations beyond ataxic and hemiplegic symptoms including cerebral and behavioral symptoms.
The careful analysis of our phenotypes does not provide useful data to elucidate the molecular mechanisms underlying the different clinical expressions of the disorder in mother and son.
Although the CACNA1A gene can generate different Cav channels by alternative pre-mRNA splicing, some of which accept been associated with SCA6 and FHM1 phenotypes, to our knowledge no alternative splicing has been identified to be the crusade of intrafamilial phenotypic variability [x, 26]. Furthermore, it has been suggested that mutations involving the splice site regions are oftentimes associated with higher variability of the phenotype and with the overlap of the common symptoms of the allelic disorders (FHM1 and SCA6) [17]; in add-on, the dissimilar expression of Ca52.1 in the several neural networks could account for the variability of symptoms among the family unit members carrying the same mutation. Finally, equally the emotional stress, minor caput trauma, concrete exercise, booze or caffeine are the most common triggers of FHM1 and EA2 attacks, other unknown individual modifier genes and / or environmental factors could play a regulator role in the phenotypic variability.
Availability of information and materials
All information generated or analyzed during current report are available from the corresponding author on reasonable asking.
Abbreviations
- HM:
-
Hemiplegic migraine
- SHM:
-
Sporadic hemiplegic migraine
- FHM:
-
Familial hemiplegic migraine
- CACNA1A:
-
Calcium channel, voltage-dependent, p/q blazon, alpha-1a subunit
- ATP1A2:
-
ATPase Na+/Thou+ transporting subunit blastoff two
- SCNA1:
-
Sodium channel, neuronal type i, alpha subunit
- EA2:
-
Episodic ataxia type 2
- SCA6:
-
Spinocerebellar clutter type 6
- EEG:
-
Electroencephalogram
- WISC-IV:
-
Wechsler intelligence scale IQ score
- NEPSY-2:
-
Developmental NEuroPSYchological Assessment
- ToM:
-
Theory of Mind
- ExAC:
-
Exome Aggregation Consortium
- gnomAD:
-
Genome Aggregation Database
- PolyPhen2:
-
Polymorphism phenotyping v2
- HSF:
-
Human splicing finder
- CT:
-
Computed tomography
- WAIS:
-
Wechsler Adult intelligence calibration IQ score
- Cav2.ane:
-
P/Q voltage-dependent calcium aqueduct
- Vineland ABS-Ii:
-
Vineland adaptive beliefs scales-2
- VGCCs:
-
Voltage-gated calcium channels
- CAG:
-
Repeated codon CAG
References
-
The International Classification of Headache Disorders 3rd edition (beta version).Cephalalgia. 2013; 33:629-808.
-
Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology. 2004;62:17–22.
-
Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic Ataxia Type-two are acquired past mutations in the Ca21 aqueduct factor CACNL1A4. Jail cell. 1996;87:543–52.
-
Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with modest polyglutamine expansions in the alpha 1A-voltagedependent calcium aqueduct. Nat Genet. 1997;15:62–9.
-
Jodice C, Mantuano Due east, Veneziano L, et al. Episodic Clutter type 2 (EA2) and spinocerebellar ataxia type six (SCA6) due to CAG repeat expansion in the CACNA1A factor on chromosome 19p. Hum Mol Genet. 1997;6:1973–8.
-
Denier C, Ducros A, Vahedi Chiliad, et al. High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology. 1999;52:1816–21.
-
Alonso I, Barros J, Tuna A, et al. Phenotypes of spinocerebellar ataxia blazon 6 and familial hemiplegic migraine caused by a unique CACNA1A missense mutation in patients from a large family unit. Arch Neurol. 2003;60:610–iv.
-
Romaniello R, Zucca C, Tonelli A, et al. A wide spectrum of clinical, neurophysiological and neuroradiological abnormalieties in a family with a novel CACNA1A mutation. J Neurol Neurosurg Psychiatry. 2010;8:840–3.
-
Pradotto 50, Mencarelli Thou, Bigoni M, et al. Episodic ataxia and SCA6 inside the same family due to the D302N CACNA1A cistron mutation. J Neurol Sci. 2016;371:81–iv.
-
Lipscombe D, Andrade A, Allen Due south. Culling splicing: functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim Biophys Acta. 1828;2013:1522–9.
-
Llinás RR, Choi S, Urbano FJ, Shin HS. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type aqueduct mutant mice. Proc Natl Acad Sci U Due south A. 2007;104:17819–24.
-
Pietrobon D. CaV2.i channelopathies. Pflugers Curvation. 2010;460:375–93.
-
Angelini C, Van Gils J, Bigourdan A, et al. Major intra-familial phenotypic heterogeneity and incomplete penetrance due to a CACNA1A pathogenic variant. Eur J Med Genet. 2019;62:103530.
-
Mantuano East, Veneziano Fifty, Spadaro M, et al. Clusters of non-truncating mutations of P/Q blazon Ca2+ channel subunit ca(v)2.1 causing episodic ataxia ii. J Med Genet. 2004;41:e82.
-
Sintas C, Carreño O, Fernàndez-Castillo North, et al. Mutation Spectrum in the CACNA1A gene in 49 patients with episodic Clutter. Sci Rep. 2017;31:2514. https://doi.org/10.1038/s41598-017-02554-x.
-
Riant F, Ducros A, Ploton C, et al. De novo mutations in ATP1A2 and CACNA1A are frequent in early-onset sporadic hemiplegic migraine. Neurology. 2010;75:967–72.
-
Nachbauer W, Nocker M, Karner E, et al. Episodic ataxia type 2: phenotype characteristics of a novel CACNA1A mutation and review of the literature. J Neurol. 2014;261:983–91.
-
Trahan EM, Mercado JM. Familial hemiplegic migraines and baseline neuropsychological testing: a case report. Headache. 2019;59:917–23.
-
Indelicato Due east, Nachbauer W, Karner Eastward, et al. The neuropsychiatric phenotype in CACNA1A mutations: a retrospective unmarried center study and review of the literature. Eur J Neurol. 2019;26:66–73.
-
Schmahmann JD, Sherman JC. The cerebellar cognitive melancholia syndrome. Brain. 1998;121:561–79.
-
Levisohn 50, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–fifty.
-
Riva D, Giorgi C. The cerebellum contributes to higher functions during development: testify from a series of children surgically treated for posterior fossa tumours. Encephalon. 2000;123:1051–61.
-
Dilekoz E, Houben T, Eikermann-Haerter K, et al. Migraine mutations impair ippocampal learning despite enhanced long-term potentiation. J Neurosci. 2015;35:3397–402.
-
Jouvenceau A, Eunson LH, Spauschus A, et al. Homo epilepsy associated with dysfunction of the brain P/Q-type calcium aqueduct. Lancet. 2001;358:801–7.
-
Imbrici P, Jaffe SL, Eunson LH, et al. Dysfunction of the encephalon calcium channel CaV2.i in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–92.
-
Chang SY, Yong TF, Yu CY, et al. Historic period and gender-dependent alternative splicing of P/Q-blazon calcium channel EF-hand. Neuroscience. 2007;145:1026–36.
Acknowledgements
Nosotros thank the child and his parents who participated in this study. We acknowledge Dr. Vincenzo Antona for his advice and support in genetic counselling. We are grateful to Mr. Brinley Thomas for his assistance in checking the translation.
Funding
The publication of the newspaper was paid thanks to funds of the grant: PSN Sicilia 2015 "Azione- five.22: "Implementation of intendance pathways for continuity of care through the management of integrated hospital-territory services in patients with rare neuromuscular diseases ALS."
Author information
Affiliations
Contributions
RN participated in the conceptualization, in writing the study and in revising and redrafting the manuscript. GP participated in the acquisition of the clinical data. GDM participated in the acquisition of EEG data. EG participated in the acquisition of genetic information. SM participated in revising the manuscript and in the interpretation of EEG data. FB participated in the acquisition and in the interpretation of the clinical data. VR participated in the acquisition and in the interpretation of the clinical data. AF participated in the conceptualization of the study and in the interpretation of the neuropsychological data. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approving and consent to participate
This study was approved by the ideals committee Palermo 1 of the "Paolo Giaccone" University Hospital of Palermo, Italy. Written informed consent for participate was obtained from each of the family unit members tested.
Consent for publication
Written informed consent for publication was obtained from each of the family members tested.
Competing interests
No author has any disharmonize of interest to declare except Filippo Brighina who is a member of the editorial board (Acquaintance Editor) of this Journal.
Additional information
Publisher'southward Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Artistic Commons Attribution four.0 International License, which permits employ, sharing, adaptation, distribution and reproduction in whatever medium or format, equally long as you give advisable credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Artistic Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article'south Creative Eatables licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, y'all will demand to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/null/i.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
Most this article
Cite this commodity
Nardello, R., Plicato, G., Mangano, K.D. et al. Two distinct phenotypes, hemiplegic migraine and episodic Ataxia type 2, acquired by a novel mutual CACNA1A variant. BMC Neurol xx, 155 (2020). https://doi.org/10.1186/s12883-020-01704-5
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12883-020-01704-five
Keywords
- CACNA1A factor
- Familial hemiplegic migraine type 1
- Episodic ataxia type2
- Cognitive affective syndrome, neuropsychology.
Source: https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-020-01704-5
0 Response to "Can You Have Cacna1a and Not Know It"
Post a Comment